2 Which of the Following Molecules Is the Strongest Acid
The forces between molecules in H 2 Oliq are greater than those in N. 172 of patients from GWPCARE1 that taking valproic acid had liver transaminase elevations.
Formulas for these compounds may be seen by Clicking Here The overall process of fatty acid synthesis is summarized for palmitic acid CH 3.

. Predict the Outcome of Organic Acid-Base Reaction Use pK a as Criterion With the knowledge of acidity and pK a we are now ready to see how to apply this information to the understanding of organic reactions from an acid-base perspective. Consider the following acidbase reaction. Large atoms like xenon are more polarizable than small atoms like helium.
Figure 10 shows how a static dipole can. Field is called polarizability. A Lithium hydroxide LiOH b hexane C 6 H 14 and c iso-butane C 4 H 10 LiOH is ionic very strong C 6 H 14 is a straight chain strong C 4 H 10 is branched weak You would rank these in order strongest to.
When the chains are longer the molecules. 1 B1 lineage with the spike D614G mutation GISAID accession ID. Fluoroantimonic acid is a mixture of hydrogen fluoride and antimony pentafluoride containing various cations and anions the simplest being H 2 F and Sb F 6This substance is a superacid that can be in excess of a quadrillion times stronger than 100 pure sulfuric acid depending on the proportion of its ingredientsIt has been shown to protonate even.
An ester bond is formed when a molecule having the carboxylic group reacts with another molecule having a hydroxyl group. Compare these three compounds. In patients from GWPCARE1 Part B the monthly frequency of seizures from baseline decreased by a median of ranged from 38 to 44 in 12-week periods.
Intermolecular Forces As the number of carbons increases in a series of fatty acids the melting point increases. In IR absorption tables signal intensities height are usually denoted by the. A molecule or atom which does not have any charges present at the end due to the reason that electrons are equally distributed and those which symmetrically cancel out each other are the non- polar molecules.
The strongest of these is the triphosphate ATP with the diphosphate and monophosphate being less powerful. In this water is a polar molecule whereas. The main type of diabetes is type 1 diabetes which is caused by insulin deficiency and type 2 diabetes which is characterized by insulin resistance The majority of diabetes is type 2 diabetes and it is a formidable challenge for public health According to the International Diabetes Federation statistics 463 million people aged 2079 years worldwide.
Polyprotic acids may be further categorized according to how many protons they can donate diprotic 2 triprotic 3 etc. B The entropy change is. Amino acid sidechains with π electrons such as phenylalanine and tryptophan are more polarizable than sidechains such as isoleucine which lack π electrons.
Commonly reported AEs diarrhoea pyrexia decreased appetite and somnolence occurred in 932 of patients. The following features will have the effect of creating a higher boiling point. 2 Cl₂O₇g 2 Cl₂g 7 O₂g A The entropy change is negative because a larger molecule Cl₂O₇ is converted into two smaller molecules Cl₂ and O₂.
The following SARS-CoV-2 isolates were obtained from nasopharyngeal swabs. Indeed many molecules behave as acids in non-aqueous solution that do not do so in aqueous. As a result a water molecule is released and the two carbons.
An ester bond or ester linkage is formed between an acid and an alcohol. In a solution a polar molecule cannot be mixed with the non-polar molecule. This is because as the number of carbons increases the chains get longer.
The carboxylic group loses its hydrogen and oxygen while the alcohol loses hydrogen of its hydroxyl group. CH 3 CN as these solvents have been widely used to measure the acid dissociation constants of organic molecules. Hydrochloric acid HCl acetic acid CH 3 CO 2 H or HOAc nitric acid HNO 3 and benzoic acid C 6 H 5 CO 2 H are all monoprotic acids.
EPI_ISL_413489 from a mildly symptomatic individual. A monoprotic acid donates only one proton or hydrogen atom per molecule to an aqueous solutionThis is in contrast to acids capable of donating more than one protonhydrogen which are called polyprotic acids. Consequently tables of IR absorptions are arranged by functional group -- it some versions these may be further subdivided to give more precise information.
The acid equilibrium problems discussed so far have focused on a family of compounds known as monoprotic acidsEach of these acids has a single H ion or proton it can donate when it acts as a Brnsted acid. For example take water and oil. Because DMSO is a stronger proton acceptor than H 2 O the acid becomes a stronger acid in this solvent than in water.
H 2 O 2 H 2 ONH 3. Therefore the same or similar functional groups in different molecules will typically absorb within the same specific frequency ranges. The following reaction is an example in Section 32If you take a closer look at the reactants and products you will find that the product side.
Which of the following describes the entropy change in the given reaction. Lauric acid C11 H23 COOH 44 C myristic acid C13 H27 COOH 58 C palmitic acid C15 H31 COOH 63 C stearic acid C17 H35 COOH 70 C.

Biological Significance Of Hydrogen Bonds In Water Hydrogen Bond Bond Study Skills

Catechol Also Known As Pyrocatechol Or 12 Dihydroxybenzene Is An Organic Compound With The Molecular Formula C6h4 Oh 2 It Chemistry Catechins Distillation

Tetryonics 102 10 Tetryonic Charge Topologies Highlight The Failings Of The Current Stick N Ball Lewis Diagrams Whe Organic Chemistry Things To Come Topology

A Lecithin Is An Example Of An Emulsifying Agent B Emulsifying Agents Stabilize Emulsions By Interacting With Both The Chemistry Notes Chemistry Adaptations

12 Bis Triethoxysilyl Ethane Is A Non Functional Silane And A Double Trialkoxysilyl Precursor It Is Used As A Plating Agent 12 Bis Chemistry Molecules Nerdy

Methanol Structure And Chemical Formula In 2021 Methylation Alcohol Chemical Formula

This Is A Molecule Of Water One Of The Main Gases That A Volcano Releases We See Most Of It As Steam Mike

This Is A Molecule Of Sulfur Dioxide One Of The Harmful Gases That A Volcano Releases It Can Be Found In The Main Plume Of Volc Sulphur Dioxide Gas Lava Flow
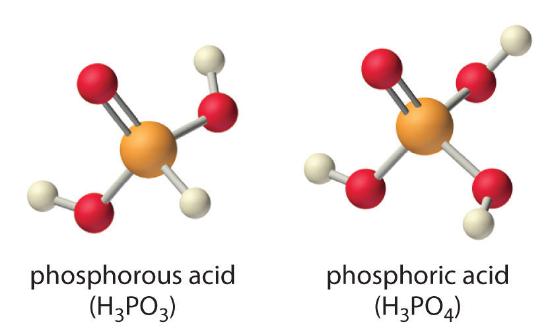
Chapter 16 3 Molecular Structure And Acid Base Strength Chemistry Libretexts

Acids Bases And Ph Scale Doodle Notes Science Doodle Notes Doodle Notes Science Science Doodles Doodle Notes

Wf5 Tungsten V Fluoride Looks Great Not Much More Is Known D Chemistry Molecule Moleculeoftheday Science Chemistrylove Chemistry Fluoride Molecules

So3 Sulfur Trioxide Molecules Toxic Chemicals Dangerous Chemicals

Novel 2 D Material Offers A Band Gap And Self Assembly Semiconductor Band Gap Space Science




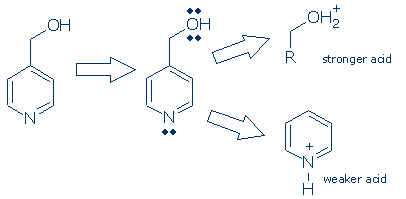


Comments
Post a Comment